Background: NHL is the 8 th most common cancer in the U.S. and exhibits poor survival, particularly among patients with disease resistant to chemotherapy. While CAR-T therapies are now available for patients with advanced disease, access to these therapies has been reported to be inequitable across varied sociodemographic populations. The Knight Cancer Institute is the only academic medical center and facility offering CART therapy in Oregon and nearby region, with the next closest center located 200 miles away. In this retrospective study, we identified baseline demographic and socioeconomic factors of NHL patients treated with CAR-T therapies at our institution, and factors associated with commercial versus investigational product used and overall survival.
Methods: Adult patients with relapsed/refractory B-cell NHL referred to the OHSU Knight Cancer Institute for CAR-T therapy between Jan 2016 and May 2023 were included. Data collected included age, gender, race/ethnicity, zip-code, insurance type, disease type and status at time of treatment, and clinical trial participation. Zip-codes were used to estimate poverty level as defined by American Community Survey, rural-urban continuum codes and distance to the treatment center.
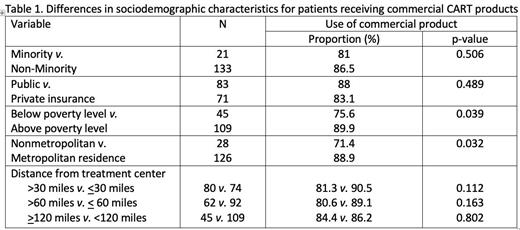
Results: A total of 154 patients with median age of 62.7 years (range, 23.5-81.6) underwent CAR-T therapy for relapsed/refractory B-cell NHL with 4 commercial (86%) and 6 investigational products (14%). Of these, 43% were older than 65 years, 68% male and 14% self-identified as non-White (including Hispanic). Approximately 66% of those younger than 65 years were privately insured, while 82% of those over 65 years had Medicare. The majority of patients were from Oregon (85%), 12% from neighboring states of Southwest Washington and Idaho, and 2.6% from states outside of the Pacific Northwest region. About 29% of patients came from areas with poverty before the national level, 18% from non-Metropolitan areas (1.3% from remote rural areas). About 52% lived more than 30 miles away and 29% lived > 120 miles from the treatment center. Of the 22 patients receiving investigational products, 59% were males, 27% were older than 65, 18% were Non-white (including Hispanic), 46% had public insurance, 29% lived in areas below the national poverty level, 36% lived in non-Metropolitan areas (9% in remote rural areas), 55% were from areas > 60 miles away and 27% from areas > 120 miles from the treatment center. There were no statistical differences in utilization of commercial vs. research products for patients from racial/ethnic minorities or those living in areas > 30 miles, 60 or 120 miles from the transplant center, as compared to their counterparts. The proportion of patients receiving commercial products (vs. investigational product) was lower for patients from nonmetropolitan areas compared to metropolitan areas (71% vs. 88%, p=0.031) and for those living in areas below the national poverty level (76% vs. 90%, p=0.039), Table 1. With a median follow-up of 303 days (range, 30-1861) for survivors, the overall survival (OS) for the entire cohort is 65.8% (95 thCI, 62.6-68.9%). There were no statistically significant differences in overall survival for patients from racial/ethnic minorities, those living in non-metropolitan areas, living > 30 miles, 60 or 120 miles from the transplant center or in areas below the national poverty level, as compared to their counterparts.
Conclusions: Although CAR-T access may vary by region, we have been able to offer this therapy to patients from socioeconomically diverse backgrounds in Oregon and nearby region. Our study highlights the importance of accounting for social determinants of health when implementing CAR-T therapy programs to optimize access to all patients. Participation in clinical trials may expand access to CART therapies, especially for those for which commercial products are less available.
Disclosures
Chen:Fate: Research Funding; Intellia: Consultancy; Kite: Consultancy, Research Funding; Elsevier: Consultancy; Novartis: Research Funding. Leonard:Pfizer: Consultancy; Adaptive Biotechnologies: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, accommodations, expenses; Takeda: Consultancy; Kite/Gilead: Consultancy. Maziarz:Athersys: Other: Patent holder; Gamida: Research Funding; Orca Therapeutics: Research Funding; Kite: Consultancy; AlloVir: Consultancy, Research Funding; Novartis: Consultancy, Research Funding. Nemecek:Novartis: Consultancy.